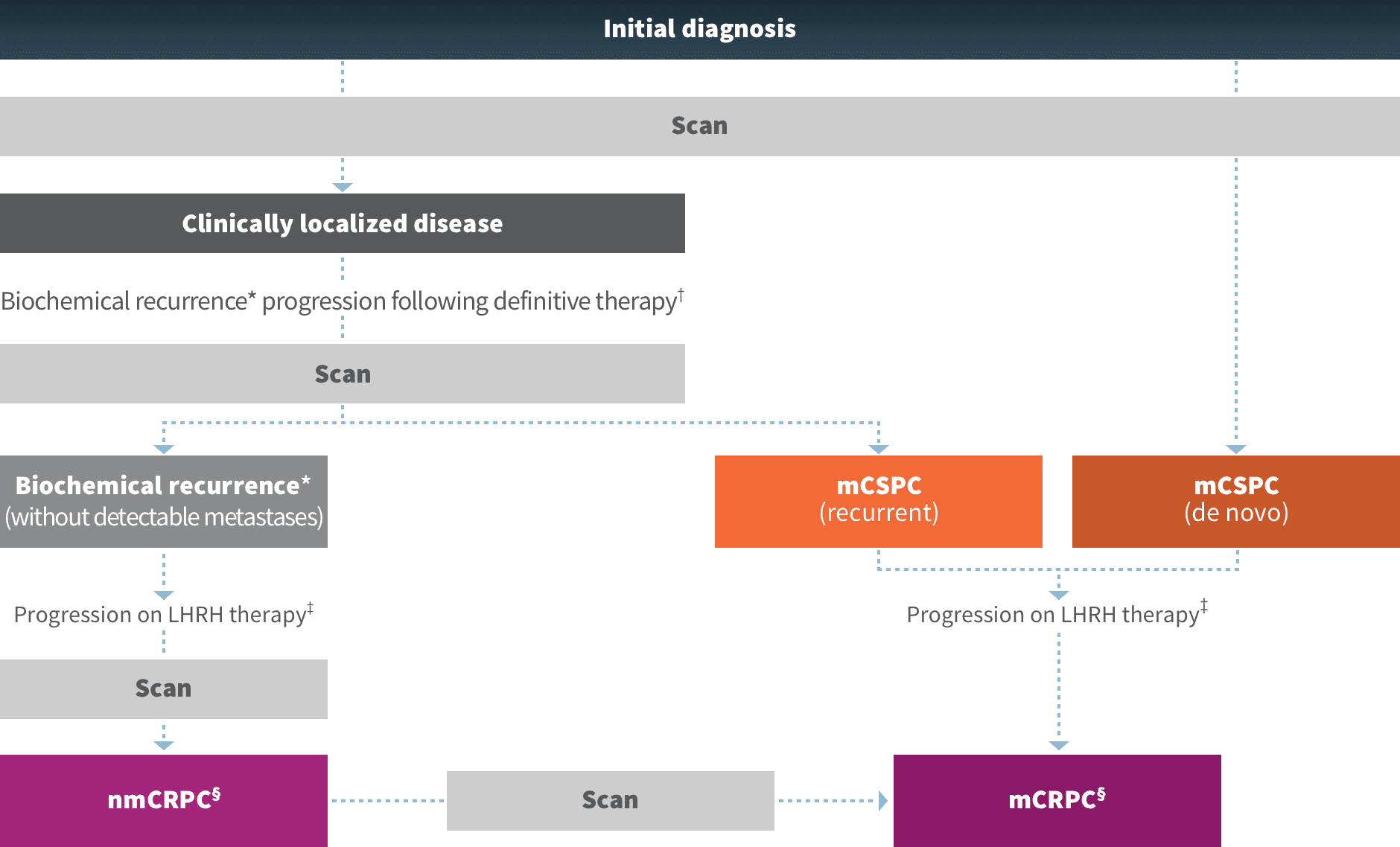
For the treatment of mCSPC
Guidelines include NHTs + ADT or chemotherapy + ADT at their highest level of recommendation1,2
1
- NHTs that are indicated for mCSPC + ADT*†
- Chemotherapy + ADT*†
2A
- External-beam radiation therapy to the primary tumor for low-volume‡ metastases + ADT
- ADT§
2B
- A select NHT + ADT
A Category 1 recommendation is based on high-level evidence and indicates uniform NCCN consensus that the intervention is appropriate.
A Category 2A recommendation is based on lower-level evidence and indicates uniform NCCN consensus that the intervention is appropriate.
A Category 2B recommendation is also based on lower-level evidence and indicates NCCN consensus that the intervention is appropriate.
Please see NCCN Guidelines for complete information.
*ADT alone or observation are recommended for asymptomatic patients with metastatic disease and a life expectancy ≤ 5 years.1
†ADT options are orchiectomy, LHRH agonist, LHRH agonist plus first-generation anti-androgen, or LHRH antagonist. Certain combinations of ADT with NHT are not recommended.1
‡High-volume disease is differentiated from low-volume disease by visceral metastases and/or ≥ 4 bone metastases, with ≥ 1 metastasis beyond the pelvic vertebral column. Patients with low-volume disease have less certain benefit from early treatment with docetaxel combined with ADT.1
§Intermittent ADT can be considered for men with castration-sensitive prostate cancer, with or without metastases, to reduce toxicity.1
Clinicians should offer
- NHTs¶ that are indicated for mCSPC + ADT
- Chemotherapy + ADT
EVIDENCE LEVEL
GRADE A
Clinicians should not offer
- First-generation anti-androgens + LHRH agonists, except to block testosterone flare
EVIDENCE LEVEL
GRADE A
Clinicians may offer
- Primary radiotherapy to the prostate + ADT in selected patients with low-volume metastatic disease
EVIDENCE LEVEL
GRADE C
A Grade A rating means the AUA is very confident that the true effect lies close to that of the estimate of the effect.
A Grade C rating means the AUA’s confidence in the effect estimate is limited. The true effect may be substantially different from the estimate of the effect.
Please see AUA Guidelines for complete information.
‖Strong recommendations are directive statements that an action should (benefits outweigh risks/burdens) or should not (risks/burdens outweigh benefits) be undertaken because net benefit or net harm is substantial.2
¶Defined as androgen pathway directed therapy.2
#Conditional recommendations are nondirective statements used when the evidence indicates that there is no apparent net benefit or harm, when benefits and harms are finely balanced, or when the balance between benefits and risks/burden is unclear.2
ADT, androgen deprivation therapy; AUA, American Urological Association; LHRH, luteinizing hormone-releasing hormone; mCSPC, metastatic castration-sensitive prostate cancer; NCCN, National Comprehensive Cancer Network; NHT, novel hormone therapy.