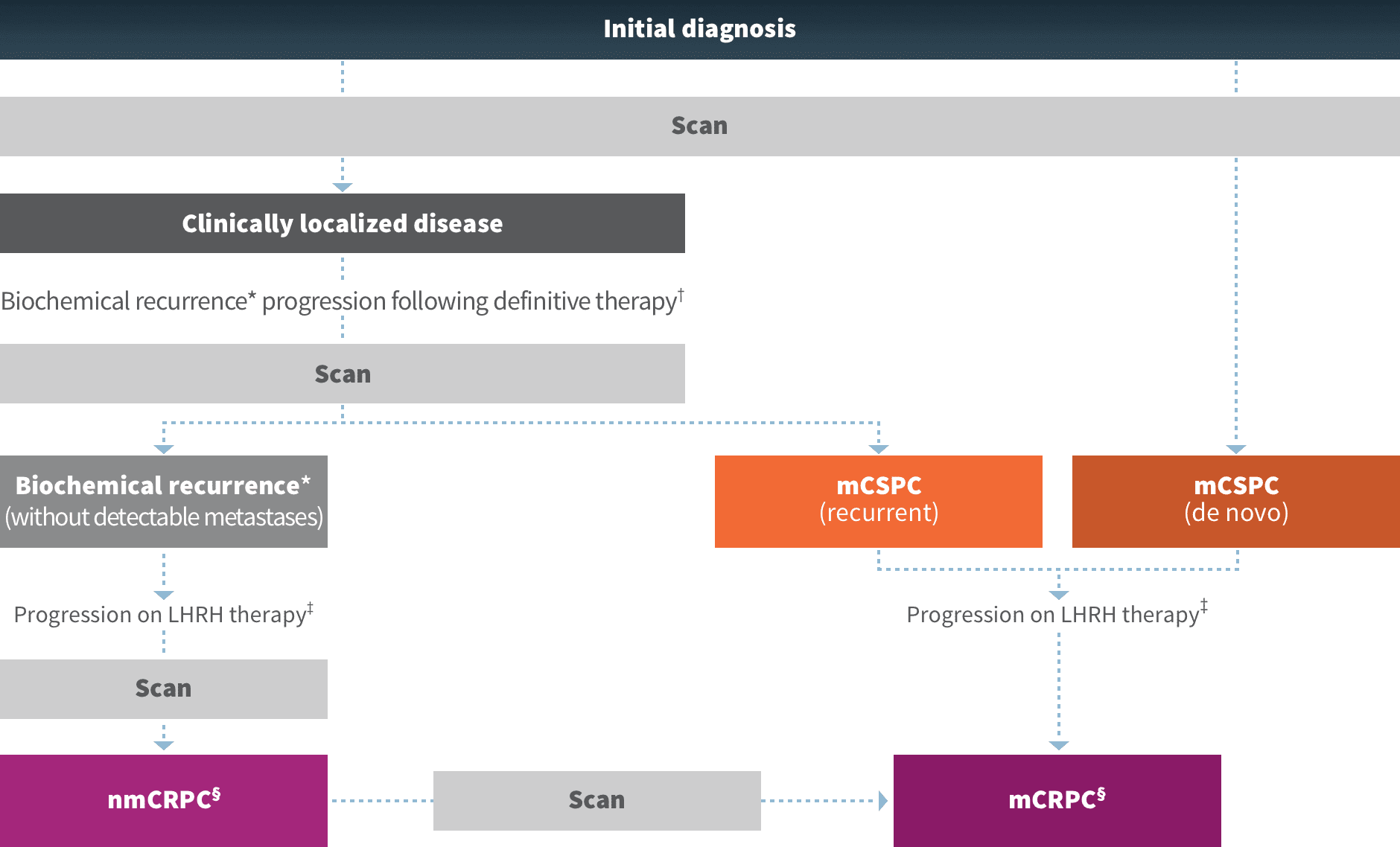
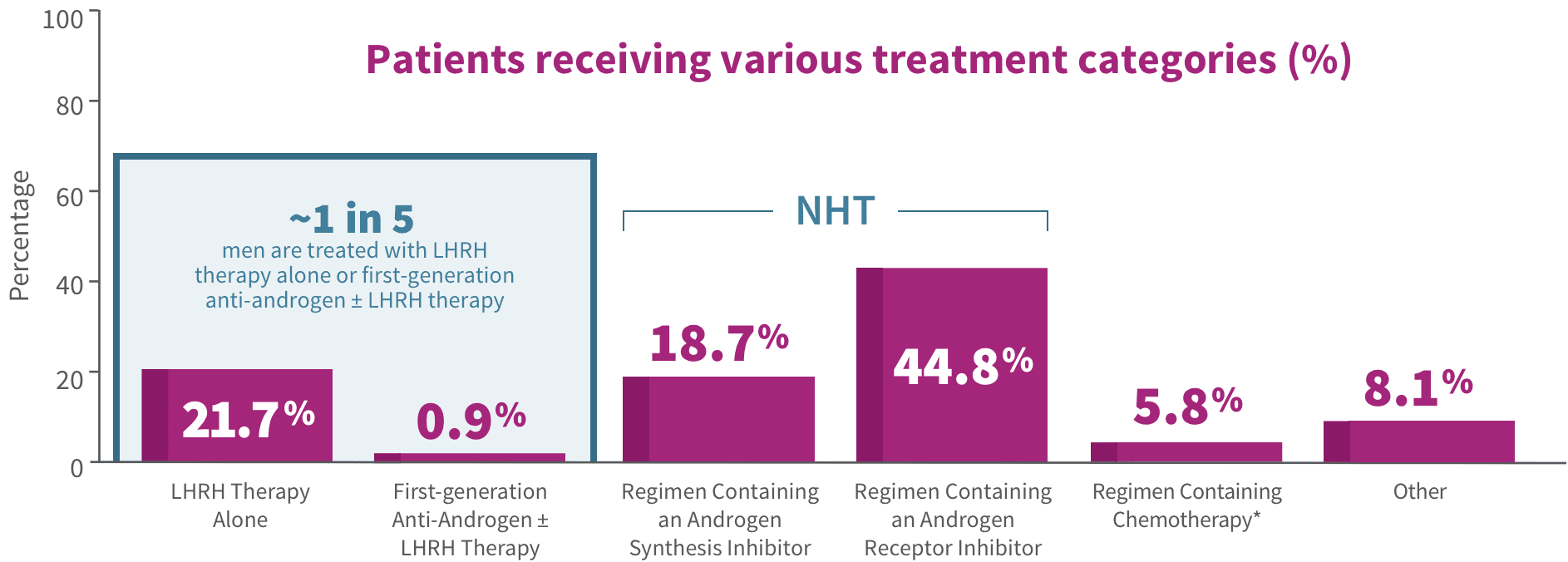
For the treatment of nmCRPC
Guidelines include NHTs + ADT at their highest level of recommendation1,2
1
- NHTs that are indicated for nmCRPC in patients with PSADT ≤ 10 months + ADT
2A
- Other secondary hormone therapy* in patients with PSADT ≤ 10 months + ADT
- Observation† or other secondary hormone therapy* in patients with PSADT > 10 months + ADT
A Category 1 recommendation is based on high-level evidence and indicates uniform NCCN consensus that the intervention is appropriate.
A Category 2A recommendation is based on lower-level evidence and indicates uniform NCCN consensus that the intervention is appropriate.
Please see NCCN Guidelines for complete information.
*Ketoconazole, ketoconazole plus hydrocortisone, first-generation anti-androgen, corticosteroids, estrogens including diethylstilbestrol, or anti-androgen withdrawal.1
†Observation involves monitoring the course of disease with the expectation to deliver palliative therapy for the development of symptoms or a change in exam or PSA that suggests symptoms are imminent.1
Clinicians should offer
- NHTs that are indicated for nmCRPC + ADT to patients at high risk for developing metastatic disease (with PSADT of ≤ 10 months)
EVIDENCE LEVEL
GRADE A
A Grade A rating means the AUA is very confident that the true effect lies close to that of the estimate of the effect.
Please see AUA Guidelines for complete information.
‡Strong recommendations are directive statements that an action should (benefits outweigh risks/burdens) or should not (risks/burdens outweigh benefits) be undertaken because net benefit or net harm is substantial.2
ADT, androgen deprivation therapy; AUA, American Urological Association; NCCN, National Comprehensive Cancer Network; NHT, novel hormone therapy; nmCRPC, nonmetastatic castration-resistant prostate cancer; PSA, prostate-specific antigen; PSADT, prostate-specific antigen doubling time.